Smart Team Building for the Shifting R&D Landscape
 By Jordan Warshafsky, Partner, Ashton Tweed
By Jordan Warshafsky, Partner, Ashton Tweed
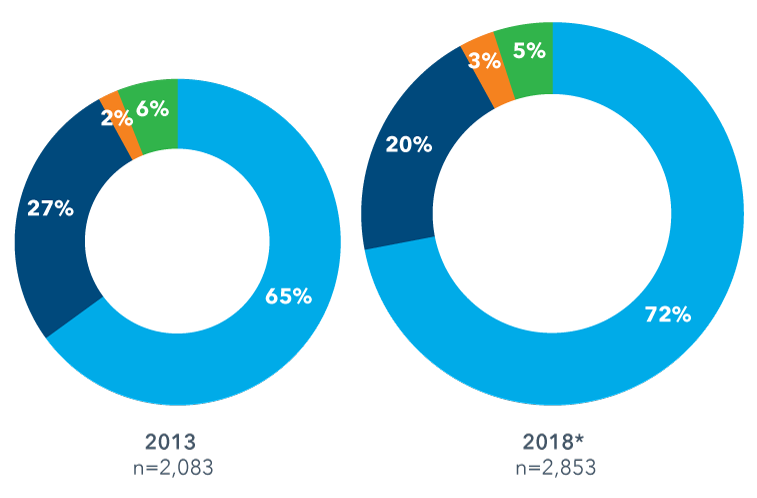
In a recent report from IQVIA, data documents the growing role of emerging biopharmas in R&D. “Emerging biopharma companies now account for over 70% of the total late stage R&D pipeline,” the report states. Just take a look at their graphic below to see the shift emerge since 2003:
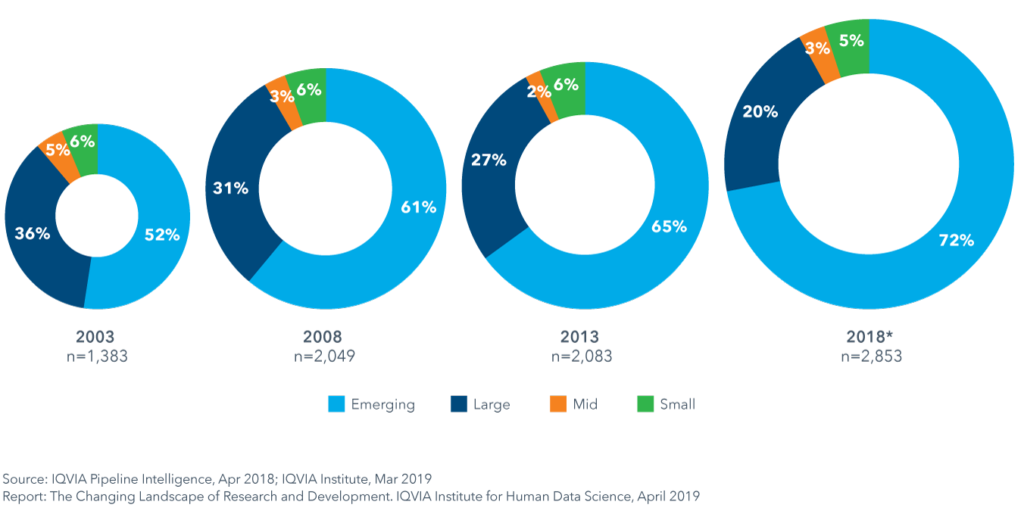
As a life science industry veteran and recruitment expert with over 35 years of experience, this trend isn’t news to me. I’ve worked in both large and small companies, and emerging biopharmas tend to do a lot of R&D legwork before getting Big Pharma involved. However, R&D has notoriously been an expensive part of the life sciences growth continuum and has been known to have a high risk of low return on investment. If emerging biopharmas are retaining control of their assets for longer and bringing them into late-stage clinical trials, it means that fundraising is playing a critical role in the success of these companies, who are working with much smaller R&D budgets than their Big Pharma counterparts.
Several other factors are making it possible for small companies to progress their innovative medicines further along the clinical pipeline, especially compared to when I was actively working in the industry as recently as the early 2000s. Technology has advanced to a degree that has allowed for much smoother communication, data collection, and sharing of information, both internally and externally. The digital revolution has made my memories of sifting through endless files and research papers obsolete. The growing focus on small patient populations and rare diseases is also making it more feasible for young companies to run smaller and more affordable clinical trials on their own. Improved data analysis tools, artificial intelligence, and even wearable devices and smartphone apps are also improving efficiency in clinical trials.
This is an exciting time for startups and small companies, but just because the trend is growing doesn’t mean that there is any less risk involved. There still exists a high failure rate of bringing new products to market in our tightly regulated industry. In order to successfully hold on to one’s pipeline for longer periods, companies need to attain sufficient funding and onboard the correct experts at the correct time. Different leadership is required to help businesses reach each milestone – to fund, kick off, and advance clinical trials. Enlisting a flexible workforce is a key hiring approach to team building, especially for smaller companies fresh out of the initial start-up phase. Management needs to be smart with their finances, as their budgets likely don’t allow for the retention of all the high-salaried employees they’d ideally have on their payroll. A strategic mix of permanent employees, interim talent, outsourced contractors, and even remote expertise will set your company up for the highest possibility of success:
Permanent Leadership: Think of this part of your team as the foundation of your company – perhaps the CEO, CFO, and scientific founders – who need to be there every step of the way to ensure that the company and product pipeline remain on track. These people are needed to address any issues that could throw your R&D off its scheduled timeline.
Interim Talent: These professionals are leaders that your company needs to help manage a short-term portion of the project; when an experienced and hands-on person is required to complete a specific task or overcome a hurdle but isn’t needed from start to finish like the permanent team members.
Outsourced Help: For certain tasks during the tumultuous R&D process, your small- to mid-sized company may need big help in the form of a CRO, consultant, university partnership, or the like. Use trusted resources like this when they offer to save you time and money on vital steps.
Remote Experts: Don’t let physical location stop you from getting the advice and insight needed in our specialized field. If your company is based in Philadelphia, but the leading expert on your contact list lives in San Francisco, that person can still provide aid remotely via virtual communication or travel.
This shift may expand beyond R&D in years to come, and I am interested in seeing how that takes shape. However, poor management is the culprit for many start-ups that go belly-up – one of the few risks that can actually be avoided in our otherwise risky industry. While setting your sights on these ambitious goals, make sure to practice smart team building to set your products up for success. For further information and true case studies on how interim talent can aid your company during clinical trials, please see this related article by my partner and CEO, Jim Rudman. Looking for advice on how to best build out your life sciences management team? Contact me today!
Share your insights! Contact jamesrudman@ashtontweed.com to contribute your life sciences article as a guest writer.





